Information for Families

Why have I been offered prenatal genomic testing?
The ultrasound scan may have identified something in your baby. Genomic testing tries to find the cause by testing genes. The result may also tell you whether the same thing could happen again.
Genomic testing can be organised after a normal chromosome microarray result from an amniocentesis or chorionic villus sampling (CVS). Genomic testing would be through an accredited medical laboratory which would provide your treating doctor with a test report when the test is completed.
Our genes and our genome
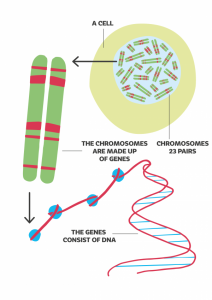
We have 23 pairs of chromosomes in our body cells which are made of DNA. Each chromosome contains many genes and each gene tells our bodies to grow and develop. We have about 20,000 genes and these are made up of DNA. Our complete DNA information is called the genome.
Changes in many genes have been linked to health conditions but we do not know what all the genes do yet. The parts of the genome outside of genes are also not understood well. All of us have changes (variations) in the DNA in our genes and most of these don’t cause health conditions. Sometimes a gene change (also called a mutation) stops the gene working as it should. They can cause health conditions. Some can cause problems in the baby during pregnancy. Gene changes may occur for the first time in the baby or may be inherited from one or both parents.
What is genomic testing?
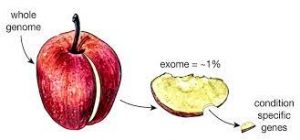
We used to be able to check one gene at a time for any faults. Now all 20,000 genes at the same time by genomic testing. This is called whole exome sequencing (WES). Testing a smaller number of genes is called panel testing. There are also large amounts of the DNA in the chromosomes that that do not make up genes. What this DNA does is still being discovered. Genomic testing all of the DNA, including the parts outside genes, is called whole genome sequencing (WGS). WGS may provide a little more information than WES.
WES and WGS tests have started to become available during pregnancies where there is a concern about the baby. The PreGen study will try to work out if WES and WGS can be useful and what is needed to provide sustainable genomic testing in pregnancy in Australia.
What is involved if I want to take part in PreGen?
You may be asked if you would like to take part in a research study (PreGen) after you have had Prenatal Genomic testing. PreGen is to help understand the best way to care for families who have genomic testing in pregnancy.
You could help if you give permission for two short questionnaires before the genomic results are ready and after 6 months. PreGen would also like to collect information from your medical record if you agree. If you give your permission, a genetic counsellor will contact you after you have had a prenatal genomic test to explain PreGen to you.
Taking part in this project is voluntary and choosing not to take part will not affect your care.
What genomic testing can be done in PreGen
PreGen is now conducting whole genome sequencing (WGS). WGS analyses all of the genetic coding in you and your baby. Testing would be done on the baby’s DNA obtained from the amniocentesis or CVS and on the DNA from blood samples collected from both parents. There will be no cost to you for the genomic testing in PreGen.
Your doctor can arrange prenatal genomic testing with a gene panel looking at a smaller number of genes, or WGS through the clinical service separately to PreGen, if that is your preference.
Taking part in PreGen is voluntary. Choosing not to take part will not affect your clinical care. Your permission will be asked to access information from your medical records and from your health professionals if you take part in PreGen.
What does participation in PreGen involve?
You will be asked to provide written consent to take part in PreGen after the CVS or amniocentesis is organised.
This permission is for you to complete a questionnaire within 4 weeks of study enrolment (before genomic results are available) and after 6 months. Information collected in the questionnaires will not be used in a way which identifies you. Information provided in the questionnaires will help us to understand family’s understanding, hopes and expectations for prenatal genomic testing.
You will also be asked for permission for healthcare professionals to collect Medicare information about the health condition identified in your the baby.
What information will I receive?
Genomic test results are usually ready within 4 weeks of starting the test, but it sometimes takes longer. A clinical geneticist and genetic counsellor will discuss the results with you. You will be told about any gene changes found in your baby that are known to cause serious health conditions in babies or children. The genomic test result may find:
• No relevant result – this means that no cause was found.
• Relevant result – This means that a cause for the problems in your baby was found. It may explain the chance of the same condition happening in future pregnancies. Sometimes this information may help your medical team look after the pregnancy, delivery or treatment in the newborn period.
• Rarely, a result related to a completely different condition that could impact on adult health and that has a treatment could be identified. This will be discussed with your doctor if it happens.
What information will not be reported?
Gene changes in your baby that could cause an untreatable adult condition will not be reported.
Sometimes, changes in genes called variants of unknown significance (VOUS) are found. A VOUS will not be reported as we do not understand whether VOUS cause disease. Sometimes additional clinical information can change a VOUS to a relevant result. This will be discussed with you if it happens.
Gene changes that could mean that someone is an unaffected carrier of a condition will also not be reported.
Where is testing done and how will information be stored?
Genetic testing will be done at NSW Health Pathology Genomics laboratories in Sydney, South Australia Pathology in Adelaide or VCGS Pathology in Melbourne. These are accredited diagnostic laboratories. Any information that can identify you will be treated as confidential by the testing laboratory and the hospital providing your care. Sometimes, de-identified information and samples may also be sent to doctors and scientists who are doing research on similar conditions. Any DNA, other tissue samples or information would be de-identified and given a unique code if researchers outside of the testing laboratory are involved.
Anonymous clinical and DNA data will be stored in Australian databases and, if required, may be shared to approved and secure international databases to improve the chance of identifying a cause for problems in babies. No identifying information will be shared into databases or with the other investigators, but details of a baby’s problems and relevant family history could be shared. Your DNA and other samples will not be used for any purpose other than the testing of genes relevant to the problems identified, without your specific written consent.
Are there any risks to me if I take part?
Sometimes, gene changes are identified that result in conditions which are not treatable in a baby, or which may cause distress during discussion. Genetic counselling support will be provided as part of your care.
Having a blood test for genomic testing for parents can cause some discomfort or bruising. For a small number of families, there may not be enough DNA sample from the amniocentesis or CVS sample and an extra sample may be needed. Sometimes, the genomic test does not work and it needs to be repeated. This would be discussed with you if necessary.
Find a Genetic Counsellor
To find a Genetic Counsellor near you click here.
More Support and Information Resources
Find out more here.
